A sodium-ion battery is a type of secondary battery that relies on the movement between the positive and negative electrodes to work, and its working principle is similar to that of lithium-ion batteries, known as a "rocking chair" battery. During charging, sodium ions are detached from the positive electrode and embedded in the negative electrode through the separator through the electrolyte, so that the positive electrode is in a sodium-poor state with high potential and the negative electrode is in a sodium-rich state with low potential. In the discharge process, on the contrary, sodium ions are removed from the negative electrode, and then re-embedded into the cathode material by the electrolyte through the diaphragm, so that the cathode is restored to a sodium-rich state. In order to maintain the charge balance, the same number of electrons are transferred through the external circuit during the charging and discharging process, and migrate together with sodium ions between the positive and negative electrodes, so that the positive and negative electrodes undergo oxidation and reduction reactions. According to the classification, sodium ions can be divided into three categories: sodium-sulfur batteries, sodium-salt batteries, and sodium-air batteries.
The development history of sodium-ion batteries
Since the theory of sodium-ion battery was proposed, it has developed in three stages: theoretical construction period, industrial recovery period and industrial explosion period. The research on sodium-ion batteries can be traced back to the 70s of the 20th century, almost simultaneously with the research on lithium-ion batteries. During this period, scientists discovered that materials such as TiS2 were able to undergo reversible electrochemical intercalation/extraction reactions with sodium ions, which marked the birth of sodium-ion batteries.
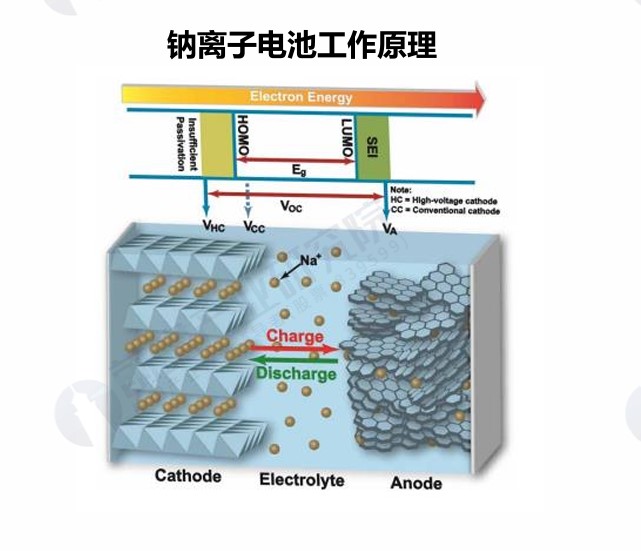
Figure: Working principle of sodium-ion battery (Source: Forward Industry Research Institute)
In the 80s of the 20th century, with the proposal of layered metal oxides as battery cathode materials, the research of sodium-ion batteries has made some progress. However, due to the low energy density of sodium-ion batteries, their development rate is slower than that of lithium-ion batteries. During this period, high-temperature sodium-ion systems such as sodium-sulfur batteries and sodium-nickel chloride batteries were developed to a certain extent. In 2000, hard carbon anode materials were discovered, which have excellent sodium-ion intercalation properties, and the research in the field of sodium-ion batteries ushered in a turning point. Since then, significant progress has been made in the research of cathode materials for sodium-ion batteries, including the development of new cathode materials such as layered metal oxides, polyanionic compounds, and Prussian blue compounds. In 2017, HiNa Battery established copper-iron-manganese positive base materials and anthracite-based anode materials to start commercial production, and in 2018, HiNa Battery realized the demonstration application of sodium ion in the world's first low-speed electric vehicle and the world's first 100kWh energy storage power station. The world's first mass-produced sodium-ion battery vehicle rolled off the production line. 2023 is regarded as the first year of the industrialization of sodium-ion batteries, and many companies have laid out the field of sodium-ion batteries, with abundant resource reserves and low cost as their important driving forces.

Figure: The development history of sodium-ion batteries
Structure of sodium-ion battery industry
The industrial chain system of sodium-ion batteries is similar to that of lithium batteries, and the manufacturing of sodium-ion batteries is compatible with the manufacture of lithium-ion batteries, and lithium-ion battery equipment can be used. The industrial structure of sodium-ion batteries can be divided into three parts: upstream raw materials, midstream battery manufacturing and downstream applications.
Upstream raw materials: including cathode materials, anode materials, electrolytes, separators, current collectors, etc. Due to the abundant resources of sodium, it is widely distributed in the sea and land.
Midstream battery manufacturing: It involves the manufacturing of batteries, including prismatic batteries, cylindrical batteries, pouch batteries, blade batteries and other different packaging forms. The production process of sodium-ion batteries is similar to that of lithium-ion batteries, and can be modified with existing lithium-ion battery production lines to accommodate the production of lithium-ion batteries. At present, the industrialization process of sodium-ion batteries is accelerating, and many companies have laid out the field of sodium-ion batteries, including CATL, HiNa Battery, Sunwonda, etc.
Downstream applications: Sodium-ion batteries can be used in low-speed electric vehicles, AGVs (automated guided vehicles), electric motorcycles, electric bicycles, smart grid energy storage and home energy storage systems. Due to the unique advantages of sodium-ion batteries in terms of safety and cost, their market prospects in energy storage and other fields are widely optimistic. With the maturity of technology and the advancement of industrialization, sodium-ion batteries are expected to be applied in more fields and promote the rapid development of related industries.
At present, the industrialization process of sodium-ion batteries is accelerating, and the enterprise layout of all links in the industrial chain is gradually improving. With technological breakthroughs and the growth of market demand, sodium-ion batteries are expected to play an important role in the future battery industry.
Related:






